1. Introduction
The morbidity and excess mortality induced by obesity, as defined by the body mass index (BMI), are of considerable concern [1,2]. In Europe, the prevalence of overweight and obesity is 31.9% and 13.9%, respectively [3], lower than in the USA (~40% and 22%, respectively).
While general practitioners focus primarily on prevention [4], pharmaceutical companies are developing new incretins [5,6], and surgeons are applying bariatric procedures to fight morbid obesity [7,8], several companies have proposed varied empirical and sometimes controversial dietary approaches [9,10,11]. More recently, modification of the gut microbiota has shown substantial benefits in overweight and obese patients [12].
Over the last ten years, the Dietplus® program has focused on the nutritional management of obesity (30 > BMI > 35) and overweight (25 < BMI < 30) without invasive procedures. This concept is based on personalized assessment, coaching, and follow-up. Food supplements validated by Belgian and European legislations [13,14] are compiled, and diets, food products, and personalized recipes and dishes are recommended.
The literature provides poor objective evidence on the effect of nutritional programs alone or in combination with the recommended phyto-derived food supplements (PDFSs) to facilitate dietary rebalancing [15,16]. Nevertheless, the benefits of human clinical trials using PDFSs have been reported [17,18,19,20,21,22,23,24,25], including improved well-being, loss of body weight, better lipid profile, fewer osteoarticular complaints, lower blood pressure, lower glycemic levels, and reduced respiratory insufficiency and cardiovascular risk. Some of these factors are directly associated with weight control and improved cholesterol profiles [26].
This study aims to assess the morphometric, behavioral, quality of life, and biological effects of a dietary rebalancing program, including potentiation with PDFSs.
2. Materials and Methods
2.1. Study Population, Inclusion Criteria and Enrollment
One hundred and seventy obese subjects (BMI > 29) were consecutively enrolled in the Dietplus® program for a period of 12 weeks on a voluntary basis. The participants agreed to adhere to the weight-control program and participate in the clinical study, including close anthropometric, behavioral, and biological evaluation. Medical history recorded prior to the Dietplus® treatment showed that only 39% of the subjects reported no medical pathology of a metabolic nature. Meanwhile, 61% suffered from associated pathologies: treated hypertension (22), disabling osteoarticular and back pathologies (10), dyslipidemia (5), treated diabetes (4), gastroesophageal reflux (3), cardiorespiratory insufficiency (1), non-alcoholic steatohepatitis (1) (NASH), and substituted hypothyroidism unrelated to obesity (8). Several obesity-related malignant neoplasms were reported, including two surgically resected breast cancers.
Simultaneously, 30 obese control subjects were recruited voluntarily during consultations with a multidisciplinary medical team for a medical work-up. These participants did not undergo therapeutic intervention or take medication during observation. They were also assessed for motivation, morphometric, and blood variations and underwent ultrasound, cardiac workup, sleep polysomnography, gastroscopy, and preoperative radiological examinations, and preoperative assessment. Furthermore, they received information about their medical condition without any therapeutic interference. None of the controls took PDFSs, nor were dietary restrictions imposed. Due to the hazards associated with the COVID-19 pandemic and the lack of knowledge regarding the drop-out rate, the study period was extended [27].
2.2. The Dietplus® Program
After a parametric and nutritional assessments and the preventive history, the Dietplus® program implements nutritional education on a low-calorie diet, personalized coaching on an adequate way of life, and regular intake of PDFS.
The nutritional educational aspect includes oral and written training in nutrition and diet, covering the basics of a balanced diet comprising proteins, carbohydrates, and lipids. Meal collection, preparation, cooking, and presentation are then reviewed. Personalized coaches are assigned to each subject for the duration of the program. These coaches provide weekly guidance to boost motivation and alter eating and sleeping patterns. The subject commits to following the complete plan, which is re-evaluated every week in accordance with the regulations in each country (monitored by an independent subcontractor: https://www.pharmanager-development.com/ (accessed on 15 May 2023)). The weekly administration of the PDFS associations is applied in the same way for each subject. The information regarding the properties follows the PDFS Belgian regulations [28]. European and national Institutions regulating the PDFS determine which claimed properties can be mentioned.
The PDFS program is changed every week. The content and the sequence of PDFS associations are classified into 3 groups: PDFSs directly linked to weight loss, providing satiety, and reducing appetite; PDFSs and food supplements for digestion and sleep; and food plans specifically developed for their dietary properties. The latter contains organic cereals based on wheat germ and various products with a low glycemic index and a high protein content. The PDFSs provided in the global program include green tea leaf, ash leaf, fennel seed, cinnamon bark, dandelion leaf, quack grass rhizome, orthosiphon leaf, black elderberry, organic rosemary leaf, elderflower, green tea dry extract, turmeric root, dandelion leaf, nopal powder, chitosan, Ascophyllum thallus, artichoke leaf, dry extract of green mate, artichoke dry extract, cola nut, dry extract of guarana, L-carnitine, chromium chloride, marshmallow leaf, oat seed, mate dry extract, guarana seed dry extract, brewer’s yeast powder, black radish root powder, vitamin B6, chromium chloride, caffeine, fructooligosaccharides, Fucus thallus, oat grain, konjac root, griffonna seed, and a multivitamin supplement. The composition of the 9 consecutive weekly programs is detailed in the (www.gastrospace.com/nutrients/, accessed on 15 May 2023).
2.3. Exclusion Criteria, Dropout, and Protocol Discontinuation
Exclusion criteria include a previous history of bariatric surgery, insulin-dependent diabetes, inflammatory bowel disease, neuropsychiatric pathology, or corticosteroid therapy. Furthermore, opposition from the attending physician made enrollment impossible. Subjects were considered to have dropped out if they refused to continue with the study protocol or if they deviated from the Dietplus® program.
The protocol anticipated three obstacles: refusal to cope with the burdensome follow-up coaching, the demands of the study protocol (digital anamnesis, blood sampling, repeated measurements), and the prolonged impact on mobility due to the COVID-19 pandemic.
2.4. Medical and Anthropometric Evaluation
Addiction to smoking or/and alcoholism was assessed. Medication (antihypertensive drugs, non-steroid anti-inflammatory drugs, hormonal substitutes, etc.) was maintained. Anthropometric measurements included age, height (cm), weight (kg), BMI (kg/m2), waist circumference (cm) (Pointer > 88 in women and > 102 in men) [29], heart rate (2 readings), and blood pressure (mmHg). Body composition was estimated by impedancemetry with inBody®270 (inBody Co. Ltd. Gangnam-gu, Seoul, Korea) [30,31].
2.5. Biological Evaluation
All biochemical tests were performed by the same laboratory (SYNLAB Heppignies, accredited laboratory under number 85261020) and included hemoglobin, hematocrit, erythrocytes, VCM, TIBC, serum iron, hydromineral balance, blood ionogram (Na+/K+/Cl−/HCO3−), Vit D, Mg++, phosphorus, chronic inflammatory state (CRP US, orosomucoid, fibrinogen), PINI index (prognostic inflammatory and nutritional index) [32], nutritional status (total protein level, albuminemia, prealbuminemia), and renal function (urea, creatinine, uric acid). Resistance to insulin and potential diabetes was assessed by fasting blood sugar, insulinemia—homeostatic model assessment of insulin resistance (HOMA) [33], and quantitative insulin sensitivity check index (QUICKI-index) [34]. QUICKI = 1/[log(I0) + log(G0)], where I0 is the fasting insulin (μU/mL), and G0 represents the fasting glucose (mg/dL). Lipid parameters included total cholesterol, triglycerides, LDL, VLDL, non-LDL, and HDL cholesterol. Cardiovascular risk (apolipoproteins B, Apolipo A/B ratio, atherosclerotic index) and liver function (PAL, GGT) were also assessed.
2.6. Behavioral Evaluation and Monitoring
Evaluation of the psycho-affective state, motivation, determination, and affective environment was evaluated by several self-assessment scales completed online by each subject. A WHO-compliant “quality of life” scale was applied before and after the protocol. Prochaska and Di Clemente developed a scale based on multiple contradictory questions [35,36] to test the subject’s ability to change. The study focused on lifestyle changes, such as consumption habits, eating habits, physical activity, and, more generally, lifestyle changes .
The Mac Gill scale [37] validated for obese patients [38] was applied. The success of any weight control program depends on the attitude toward food, particularly the organization of meals, food preparation, and “conscientiousness” [39,40,41,42]. Indeed, obesity alters taste, and relative anosmia favors obesity [43,44]. The subjects were submitted to a 20-item nutritional questionnaire, “Nutriscore.” The score, calculated on a scale of 60, bears no relation to the one used in food chain labeling [45] .
Daily physical activity was assessed using a 15-question online questionnaire focusing on four areas of daily life: private and professional spheres, leisure, and structured sports activity . Only 74 subjects who responded to all four online questionnaires were considered for data collection.
2.7. Statistical Analysis
Statistical analysis was performed using R Statistical Software (v4.2.2; R Core Team 2022). Data were presented as median (range) or mean ± standard deviation (SD) for continuous variables according to non-normal or normal distribution (determined by the Shapiro–Wilk test), respectively. For categorical variables, data were presented as numbers (percentages), whereas continuous variables, pre- and post-treatment data were compared using a Wilcoxon signed-rank test for non-normal distributions or a Student’s t-test for paired samples with normal distribution. The threshold for statistical significance was set at p < 0.05. Effect sizes were calculated as follows: Cohen’s d (1988) for parametric tests (small effect: d ≥ 0.2; mean effect: d ≥ 0.5; large effect: d ≥ 0.8 and below 0.2 is trivial), and rank-biserial correlation for non-parametric tests.
3. Results
The program compliance of the 200 subjects is illustrated in Figure 1.
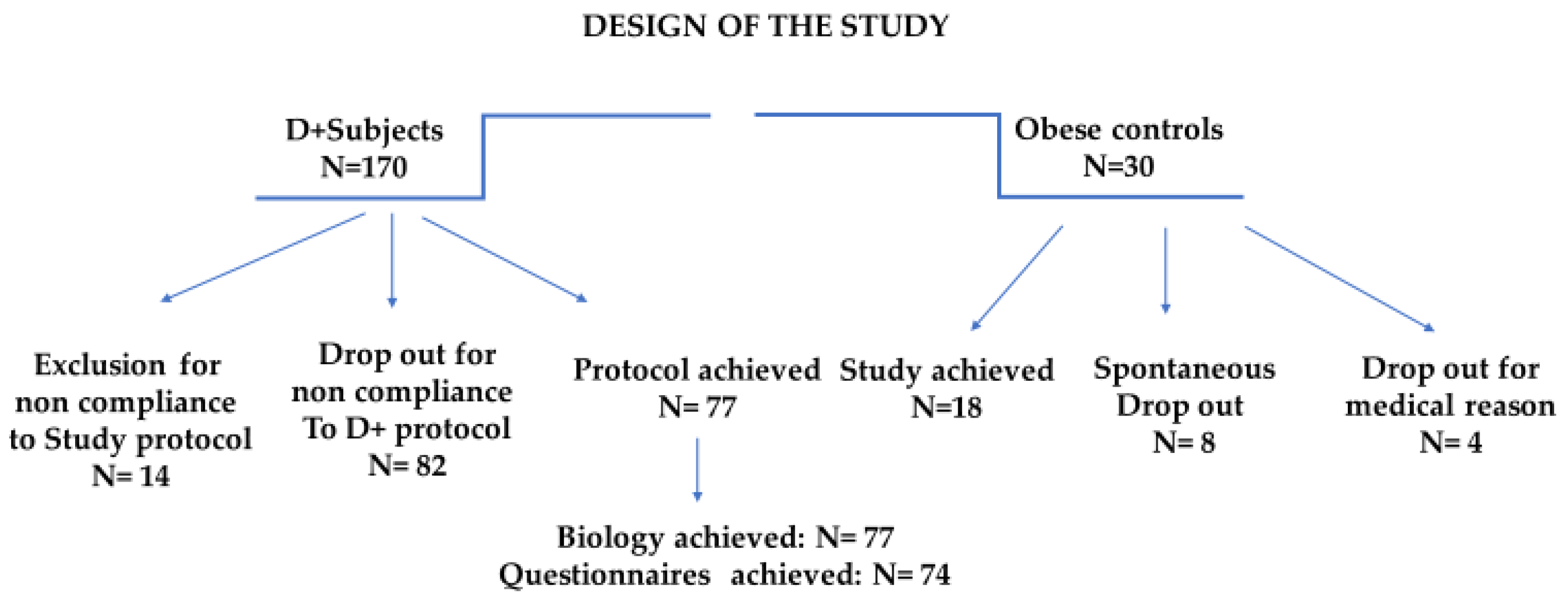
Figure 1. Study design focusing on the clinical pathways and incidence of dropout and exclusion.
3.1. Dropout: Analysis and Interpretation
The protocol interruptions in Dietplus® and control subjects are presented for both groups. Of the 30 controls, 5 did not follow the clinical itinerary, and 4 had a pathology contraindicating the continuation of the bariatric surgery protocol (neuroendocrine tumor, pregnancy, acute diverticulitis, hepatic mass). Finally, family constraints, financial issues, and a lack of compliance with the study protocol impacted one patient each.
Protocol interruptions for Dietplus® and control subjects were comparable. The investigators called back the 95 subjects excluded or lost to follow-up; 15 could not be contacted or refused to be interviewed. Meanwhile, 80 explained their failure, revealing five major causes and four more anecdotal causes.
Among Dietplus® subjects, the five main reasons for abandonment include a severe lack of compliance with the Dietplus® program (26.2%), medical exclusion criteria (15%), family constraints with the predominance of strong opposition from a life partner, financial constraints (weekly cost was estimated between 40 and 50 €) and logistical problems often related to professional constraints.
3.2. Comparison of the Two Cohorts
We sought to determine whether the profile of control patients differed from that of the Dietplus® customers. The profiles were similar in age, sex, BMI, and abdominal obesity. Nevertheless, the Dietplus® subjects dropped out more often, had fewer co-morbidities, and a lower weight-loss target (−19 kg) than the control group (−28 kg).
At the family, behavioral, and psychological levels, the two cohorts were comparable, with even alcohol consumption and smoking identical. Moreover, upon enrollment, the Dietplus® subjects had an average height of 165.1 ± 11 cm and mean weight of 94.1 ± 14.2 kg, with extremes ranging from 73 to 158 kg. Regarding abdominal obesity, the waist circumference in the 148 women was 108.4 ± 12 cm, categorized as pathological for 77 subjects.
The two cohorts were compared at the time of enrollment regarding psycho-behavioral scores . Notably, control patients were referred for medical treatment, whereas Dietplus® subjects sought a non-medical approach. While the quality of life and sporting activities were identical in both cohorts, dietary and nutritional behavior was significantly (p < 0.01) more disturbed in Dietplus® subjects, with a nutriscore of 43.5 ± 4.2% versus 59 ± 5.7%. However, the ability to change (Prochaska Di Clemente Scale) was significantly improved (p < 0.01) when the Dietplus® program was considered.
The two cohorts presented reasonably comparable profiles, with higher expectations in obese patients consulting a medical team. Eating habits were more compromised in subjects within the Dietplus® group.
3.3. Evolution of the Control Group
The slight weight gain observed in the control group expected as obese patients are more likely to seek medical attention during periods of weight regain. During the work-up and observation periods, the weight gain was 1.0 ± 0.2 kg. The results were expected given that no therapeutic action was applied in the controls. However, the medical and paramedical consultations led to “awareness” as ¼ of patients moved from the contemplation stage to the determination stage.
The 12 weeks of medical observation and communication of the results of the medical work-up did not improve quality of life, nutritional habits, or physical activity. The only observed impact of the medical work-up was positive changes in motivation.
3.4. Effect of The Dietplus® Cure
The effect of the program was measured via pre- and post-therapeutic values. Nevertheless, the comparison with the control group was useful as the natural evolution of obesity was unfavorable during the same period. For example, at the end of the Dietplus® program, subjects lost an average of 10 ± 2.2 kg compared to the control group whose overweight increased.
4. Discussion
This study exhibited a major drop-out rate in both study groups. Hence, helping obese individuals control their diet and energy expenditure remains a major challenge [46,47,48,49].
4.1. Common Clinical Problems Observed in Obese Subjects
In the control population, almost one-third of patients dropped out during the development process. In this instance, the phenomenon of medical shopping is emphasized as obese patients often consider being overweight as a cosmetic problem [50]. Such a high drop-out rate was observed in both cohorts. This underscores the difficulty in motivating changes in dietary behavior and incorporating physical activity.
The certainty of knowing one’s solution to the problem further complicates the relationship with any therapist. Moreover, the physician builds less rapport with obese patients [51]. Indeed, the weight curve during the study period exhibited a natural deterioration in the control group rather than a stable weight. However, in this group, biochemical values remained stable during the 12-week observation period.
4.2. Morphometric Parameters
Substantial weight loss was observed in the Dietplus® subjects. All measurements, in particular impedancemetry, confirmed a reduction in abdominal obesity, with positive consequences on vital parameters, namely, a decrease in heart rate, hypertension, and cardiovascular risk [52,53]. Physical indices of comorbidity risk were also clearly improved. However, this study is limited by a relatively short-term follow-up. Hence, systematic annual monitoring of the 77 subjects who had completed the protocol is underway.
4.3. Behavioral Parameters
The Dietplus® program induced positive effects on the behavior of obese subjects, with improved QOL, Nutriscore, motivation to change lifestyle, and (discrete) improvement in physical activity. The necessity of adherence to a strict protocol is confirmed [46,54].
4.4. Metabolic Parameters
Several metabolic parameters were significantly improved over the 12-week study period, certainly in relation to the average 9 kg weight loss.
5. Conclusions
Healthcare systems are facing a global overweight and obesity pandemic. While many non-invasive methods lack scientific evaluation and evidence-based support, the present study provides evidence of a clear benefit for the Dietplus® program. However, the impact of this study is limited by several constraints, including the absence of a double-blind controlled procedure and the concept of a totum program with different modes of action, excluding physical training [95]. Indeed, a substantial improvement in metabolic parameters and behavioral changes were observed that are likely to guarantee long-term weight control. Moreover, the contribution of the Dietplus® program could be considered in terms of cost-effectiveness [96] within the panel of therapeutic approaches to weight control.
References
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of life lost due to obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Drieskens, S. Etat Nutritionnel. Enquête de Santé 2018. Sciensano. Available online: https://www.sciensano.be/en/health-topics/obesity/numbers#overweight-and-obesity-in-belgium (accessed on 15 May 2023).
- Aceves-Martins, M.; López-Cruz, L.; García-Botello, M.; Gutierrez-Gómez, Y.Y.; Moreno-García, C.F. Interventions to Prevent Obesity in Mexican Children and Adolescents: Systematic Review. Prev Sci. 2022, 23, 563–586. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Wu, H.X.; Hu, N.; Zhou, Y.H.; Li, L.; Xiao, F.; Wang, T.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus—A systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13435. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Closset, J.; Mehdi, A.; Barea, M.; Buedts, K.; Gelin, M.; Houben, J.J. Results of silastic ring vertical gastroplasty more than 6 years after surgery: Analysis of a cohort of 214 patients. Obes. Surg. 2004, 14, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- Cobiac, L.; Vos, T.; Veerman, L. Cost-effectiveness of Weight Watchers and the Lighten Up to a Healthy Lifestyle program. Aust. N. Z. J. Public 2010, 10, 16–23. [Google Scholar] [CrossRef]
- Thorning, T.K.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Weight loss and weight loss maintenance efficacy of a novel weight loss program: The retrospective RNPC® cohort. Obes. Med. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6780. [Google Scholar] [CrossRef]
- Directive 2002/46/CE du Parlement Européen et du Conseil du 10 Juin 2002 Relative au Rapprochement des Législations des États Membres Concernant les Compléments Alimentaires (Version Consolidée du 20 Mars 2021). Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/directive_2002_46_fr_consol_3_2021_0.pdf (accessed on 15 May 2023).
- Saper, R.B.; Eisenberg, D.M.; Phillips, R.S. Common dietary supplements for weight loss. Am. Fam. Phys. 2004, 70, 1731–1738. [Google Scholar]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A.A. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Messina, V.; Rowland, I.; Frankowska, A.; Bradbury, J.; Smetana, S.; Medici, E. Plant-Based Dairy Alternatives Contribute to a Healthy and Sustainable Diet. Nutrients 2023, 15, 3393. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.A.; Granner, M.L.; Conway, J.M.; Ainsworth, B.E.; Dobre, M. Availability of weight-loss supplements: Results of an audit of retail outlets in a southeastern city. J. Am. Diet. Assoc. 2006, 106, 2045–2051. [Google Scholar] [CrossRef]
- Ngondi, J.L.; Etoundi, B.C.; Nyangono, C.B.; Mbofung, C.M.; Oben, J.E. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo-controlled investigation. Lipids Health Dis. 2009, 8, 7. [Google Scholar] [CrossRef]
- Onakpoya, I.; Davies, L.; Posadzki, P.; Ernst, E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: A systematic review of randomized controlled trials. J. Diet Suppl. 2013, 10, 29–38. [Google Scholar] [CrossRef]
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: An update. Obes Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef]
- Manore, M.M. Dietary supplements for improving body composition and reducing body weight: Where is the evidence? Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 139–154. [Google Scholar] [CrossRef]
- Icken, D.; Feller, S.; Engeli, S.; Mayr, A.; Müller, A.; Hilbert, A.; de Zwaan, M. Caffeine intake is related to successful weight loss maintenance. Eur. J. Clin. Nutr. 2016, 70, 532–534. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Randell, R. Fat burners: Nutrition supplements that increase fat metabolism. Obes. Rev. 2011, 12, 841–851. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R. Impact of the COVID-19 pandemic on clinical research. Nat. Rev. Nephrol. 2020, 16, 562–564. [Google Scholar] [CrossRef]
- AFSCA Allégations Nutritionnelles et de Santé. Bulletin 2014, 58, 1–12. Available online: https://www.favv-afsca.be/viepratique/allegationsnutritionnelles/dossier/ (accessed on 15 May 2023).
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Podstawski, R.; Omelan, A.; Borysławski, K.; Wąsik, J. Relationships between anthropometric and body composition characteristics and age in Polish women over 60 as affected by their socioeconomic and health status and physical activity levels. Front. Physiol. 2023, 14, 1198485. [Google Scholar] [CrossRef]
- Alwash, S.M.; McIntyre, H.D.; Mamun, A. The association of general obesity, central obesity and visceral body fat with the risk of gestational diabetes mellitus: Evidence from a systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Carpentier, Y.A. A prognostic inflammatory and nutritional index scoring critically ill patients. Int. J. Vitam. Nutr. Res. 1985, 55, 91–101. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Yue, L.Q.; Katz, A.; Quon, M.J. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E804–E812. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change. Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C. Stages of change in the modification of problem behaviors. Prog. Behav. Modif. 1992, 28, 183–218. [Google Scholar] [PubMed]
- Cohen, S.R.; Russell, L.B.; Leis, A.; Shahidi, J.; Porterfield, P.; Kuhl, D.R.; Gadermann, A.M.; Sawatzky, R. More comprehensively measuring quality of life in life-threatening illness: The McGill Quality of Life Questionnaire—Expanded. BMC Palliat. Care 2019, 18, 92. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Soave, F.; Ruocco, A.; Dametti, L.; Calugi, S. Quality of Life and Physical Performance in Patients with Obesity: A Network Analysis. Nutrients 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.; Britton, P.J.; Logue, E.; Smucker, W.; Milo, L. The relationship among the transtheoretical model of behavioral change, psychological distress, and diet attitudes in obesity: Implications for primary care intervention. J. Clin. Psychol. Med. Settings 1995, 2, 249–267. [Google Scholar] [CrossRef]
- Pietrabissa, G.; Sorgente, A.; Rossi, A.; Simpson, S.; Riva, G.; Manzoni, G.M.; Prochaska, J.O.; Prochaska, J.M.; Cattivelli, R.; Castelnuovo, G. Stages of change in obesity and weight management: Factorial structure of the Italian version of the University of Rhode Island Change Assessment Scale. Eat Weight Disord. 2017, 22, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Carrard, I.; Kruseman, M.; Chappuis, M.; Schmutz, N.; Volery, M. Un outil pour évaluer les comportements alimentaires: ESSCA [A tool for assessing eating behaviors: ESSCA]. Rev. Med. Suisse 2016, 12, 591–596. (In French) [Google Scholar]
- Balani, R.; Herrington, H.; Bryant, E.; Lucas, C.; Kim, S.C. Nutrition knowledge, attitudes, and self-regulation as predictors of overweight and obesity. J. Am. Assoc. Nurse Pract. 2019, 31, 502–510. [Google Scholar] [CrossRef]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Hercberg, S.; Touvier, M.; Salas-Salvado, J. Group of European scientists supporting the implementation of Nutri-Score in Europe. The Nutri-Score nutrition label. Int. J. Vitam. Nutr. Res. 2022, 92, 147–157. [Google Scholar] [CrossRef]
- Burgess, E.; Hassmén, P.; Pumpa, K.L. Determinants of adherence to lifestyle intervention in adults with obesity: A systematic review. Clin. Obes. 2017, 7, 123–135. [Google Scholar] [CrossRef]
- Monnier, L.; Schlienger, J.L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2021, 47, 101192. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C.; Kenny, P.J. Food addiction: A valid concept? Neuropsychopharmacology 2018, 43, 2506–2513, Erratum in Neuropsychopharmacology 2019, 44, 834. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Dalle Grave, R.; Mannucci, E.; Molinari, E.; Compare, A.; Cuzzolaro, M.; Marchesini, G. Complexity of attrition in the treatment of obesity: Clues from a structured telephone interview. Int. J. Obes. Lond. 2006, 30, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Shabana, H.S. Obesity, More than a ‘Cosmetic’ Problem. Current Knowledge and Future Prospects of Human Obesity Genetics. Biochem. Genet. 2016, 54, 1–28. [Google Scholar] [CrossRef]
- Gudzune, K.A.; Beach, M.C.; Roter, D.L.; Cooper, L.A. Physicians build less rapport with obese patients. Obes. Silver Spring 2013, 21, 2146–2152. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Zhang, C.; Rexrode, K.M.; van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years of follow-up in US women. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Di Clemente, C.C. Toward a Comprehensive Model of Change. In Treating Addictive Behaviors: Processes of Change; Miller, W.R., Heather, N., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 3–27. [Google Scholar]
- King, R.I.; Florkowski, C.M.; Yeo, J.; Walmsley, T.A.; Shand, B.I.; Scott, R.S.; George, P.M. What is the best predictor of the atherogenic LDL subclass phenotype ‘pattern B’ in patients with type 2 diabetes mellitus? Ann. Clin. Biochem. 2011, 48 Pt 2, 166–169. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121 Pt A, 24–41. [Google Scholar] [CrossRef]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83 (Suppl. S6), 1499S–1504S. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Gamma-glutamyl transferase and cardiovascular disease. Ann. Transl. Med. 2016, 4, 481. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Bouzas, C.; Mascaró, C.M.; Casares, M.; Llompart, I.; Abete, I.; Angullo-Martinez, E.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 2223. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F. Villa Pini Stroke Data Bank Investigators. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke 2002, 33, 1763–1771. [Google Scholar] [CrossRef]
- Neves, C.V.B.; Mambrini, J.V.M.; Torres, K.C.L.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Lima-Costa, M.F.; Peixoto, S.V. Association of metabolic syndrome with inflammatory markers in a sample of community-dwelling older adults. Cad. Saude Publ. 2019, 35, e00129918. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Med. Kaunas 2019, 55, 541. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; McAllister, M.J.; Huang, C.J. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflamm. Res. 2015, 64, 565–575. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- EFSA. Vitamine D: L’EFSA Définit des Valeurs Nutritionnelles de Référence pour la Vitamine D. Available online: https://www.efsa.europa.eu.oct.2016 (accessed on 15 May 2023).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H.; Entezari, M.H. Effect of Vitamin D Supplementation on Weight Loss, Glycemic Indices, and Lipid Profile in Obese and Overweight Women: A Clinical Trial Study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, N.J. Atténuer la carence épidémique en vitamine D: La tourmente des données scientifiques. Can. Fam. Phys. 2011, 57, e1–e6. (In French) [Google Scholar]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Contento, I.R. Nutrition education: Linking research, theory, and practice. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 176–179. [Google Scholar]
- Van Rinsum, C.; Gerards, S.; Rutten, G.; Johannesma, M.; van de Goor, I.; Kremers, S. The implementation of the coaching on lifestyle (CooL) intervention: Lessons learnt. BMC Health Serv. Res. 2019, 19, 667. [Google Scholar] [CrossRef]
- Rice, K.G.; Jumamil, R.B.; Jabour, S.M.; Cheng, J.K. Role of Health Coaches in Pediatric Weight Management. Clin. Pediatr. Phila. 2017, 56, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Nicklas, B.J. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front. Biosci. Schol. Ed. 2011, 3, 168–177. [Google Scholar] [CrossRef]
- John, N.A.; John, J.; Tarnikanti, M.; Kalpana, M.; Kamble, P.; Singhal, A.; Ganji, V.; Gaur, A.; Umesh, M.; Katta, R.; et al. Implications of lifestyle medicine in medical practice. J. Fam. Med. Prim. Care. 2023, 12, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Andreyeva, T.; Long, M.W.; Henderson, K.E.; Grode, G.M. Trying to lose weight: Diet strategies among Americans with overweight or obesity in 1996 and 2003. J. Am. Diet. Assoc. 2010, 10, 535–542. [Google Scholar] [CrossRef]
- Astell, K.J.; Mathai, M.L.; Su, X.Q. Plant extracts with appetite suppressing properties for body weight control: A systematic review of double blind randomized controlled clinical trials. Complement. Ther. Med. 2013, 21, 407–416. [Google Scholar] [CrossRef]
- Allison, D.B.; Fontaine, K.R.; Heshka, S.; Mentore, J.L.; Heymsfield, S.B. Alternative treatments for weight loss: A critical review. Crit. Rev. Food Sci. Nutr. 2001, 41, 1–28; discussion 39–40. [Google Scholar] [CrossRef]
- Rodondi, P.Y.; Degoumois, F.; Marques-Vidal, P.; Rodondi, N. Peut-on abaisser son taux de cholestérol avec des compléments alimentaires? [Is it possible to decrease cholesterol levels with dietary supplements?]. Rev. Med. Suisse 2016, 12, 451–453. (In French) [Google Scholar] [PubMed]
- Egras, A.M.; Hamilton, W.R.; Lenz, T.L.; Monaghan, M.S. An evidence-based review of fat modifying supplemental weight loss products. J. Obes. 2011, 2011, 297315. [Google Scholar] [CrossRef]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Welch, S. Comparing effectiveness of fat burners and thermogenic supplements to diet and exercise for weight loss and cardiometabolic health: Systematic review and meta-analysis. Nutr. Health 2021, 27, 445–459. [Google Scholar] [CrossRef]
- Grohmann, T.; Litts, C.; Horgan, G.; Zhang, X.; Hoggard, N.; Russell, W.; de Roos, B. Efficacy of Bilberry and Grape Seed Extract Supplement Interventions to Improve Glucose and Cholesterol Metabolism and Blood Pressure in Different Populations—A Systematic Review of the Literature. Nutrients 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Polansky, M.M.; Sato, Y.; Adeli, K.; Anderson, R.A. Cinnamon extract inhibits the postprandial overproduction of apolipoprotein B48-containing lipoproteins in fructose-fed animals. J. Nutr. Biochem. 2009, 20, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2013, 28, CD003335. [Google Scholar] [CrossRef]
- Mhurchu, C.N.; Poppitt, S.D.; McGill, A.T.; Leahy, F.E.; Bennett, D.A.; Lin, R.B.; Ormrod, D.; Ward, L.; Strik, C.; Rodgers, A. The effect of the dietary supplement, Chitosan, on body weight: A randomised controlled trial in 250 overweight and obese adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1149–1156. [Google Scholar] [CrossRef]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef]
- Emamat, H.; Zahedmehr, A.; Asadian, S.; Nasrollahzadeh, J. The effect of barberry (Berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: A randomized controlled trial. BMC Complement. Med. Ther. 2022, 22, 59. [Google Scholar] [CrossRef]
- Greenway, F.; de Jonge-Levitan, L.; Martin, C.; Roberts, A.; Grundy, I.; Parker, C. Dietary herbal supplements with phenylephrine for weight loss. J. Med. Food 2006, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-Suppressing and Satiety-Increasing Bioactive Phytochemicals: A Systematic Review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef]
- Chin, Y.H.; Ng, C.H.; Chew, N.W.; Kong, G.; Lim, W.H.; Tan, D.J.H.; Chan, K.E.; Tang, A.; Huang, D.Q.; Chan, M.Y.; et al. The placebo response rate and nocebo events in obesity pharmacological trials. A systematic review and meta-analysis. E Clin. Med. 2022, 54, 101685. [Google Scholar] [CrossRef]
- Stephens, S.K.; Cobiac, L.J.; Veerman, J.L. Improving diet and physical activity to reduce population prevalence of overweight and obesity: An overview of current evidence. Prev. Med. 2014, 62, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Verghese, N.R. Incremental cost-effectiveness of evidence-based non-surgical weight loss strategies. Clin. Obes. 2019, 9, e12294. [Google Scholar] [CrossRef] [PubMed]

